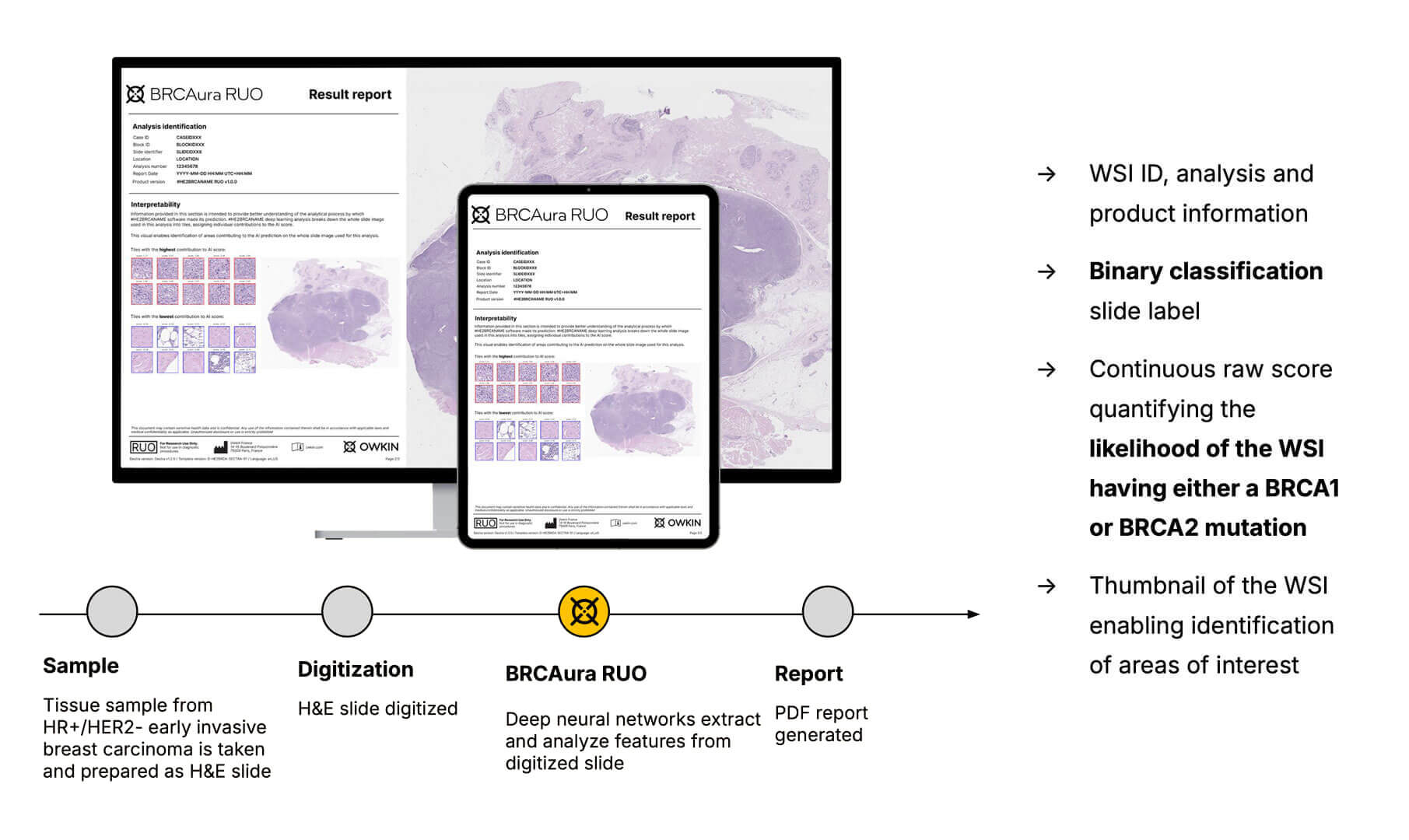
A histology model to predict germline BRCA1/2 mutations from HR+/HER2- breast cancer images
Context
Breast cancer is the leading cause of cancer death among women worldwide. germline BRCA mutation status greatly informs the therapeutic pathway and patient outcomes, but it is not universally tested in all subgroups. Expanding gBRCA testing could make targeted therapies for this biomarker more accessible to more patients. BRCAura RUO is the first AI product of its kind to screen for gBRCA status directly from H&E slides in a clinical studies setting.
Methods
Owkin developed BRCAura RUO, an AI pathology product to support gBRCA mutation testing through analysis of morphological characteristics found in digitized H&E slides from HR+/HER2- breast cancer patients.
The training set included 639 patients with a 50% gBRCA mutation prevalence. External validation was performed on three independent and international cohorts, including multiple scanners and staining types1.
Results
BRCAura RUO demonstrated strong performance in predicting gBRCA status from histology features, achieving a mean AUC of 0.80 (0.03) and mean sensitivity/specificity of 0.93 (0.01)/0.4 (0.03) in external validation cohorts. The model also showed robustness across different stains and scanners1.
Impact
With 93% sensitivity, BRCAura RUO effectively analyzes the gBRCA status from digitized H&E slides. This AI-powered prescreening approach aims to boost workflow efficiency in research settings and clinical studies while providing a better understanding of the morphological aspects of gBRCA mutations and their effects on tumor presentation.
With further validation and clinical certification, BRCAura could, at once, expand access to gBRCA testing and reduce the need for genetic testing across the eligible population. By ruling out approximately 40% of cases, BRCAura could enable more targeted molecular testing for an enriched pool of patients more likely to have the gBRCA mutation1.
1. Tchita, O. et al. Development and validation of BRCAura: A histology model to predict germline BRCA1/2 mutations from HR+/HER2- breast cancer images. ESMO 2025 published poster 327P.
This product is for Research Use Only. Not for use in diagnostic procedures. Please contact Owkin for more information. Images shown may represent the range of products, or be for illustration purposes only, and may not be an exact representation of the productManufacturer: Owkin France. BRCAura® is a trademark of Owkin Inc
BRCAura RUO is developed within the PortrAIt consortium, a french consortium financed by the government within the framework of France 2030 and by the European Union - Next Generation EU within the framework of the France Relance Plan.